Clinical Research
(CR-015) Clinical Evaluation of a Novel Intrarectal Device versus Balloon Intrarectal Device for Fecal Incontinence
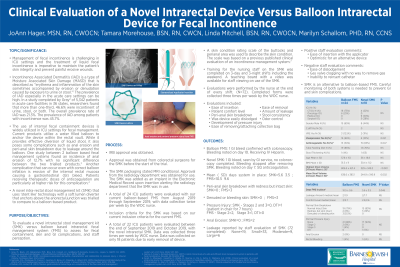
Introduction: Management of diarrhea is important in intensive care unit (ICU) patients to maintain skin integrity. However, patients are at risk for skin and gastrointestinal (GI) complications with intrarectal fecal management systems. The purpose of the project was to evaluate a novel intrarectal stool management kit (SMK) versus balloon based intrarectal fecal management system (FMS) to assess for fecal containment, skin and GI complications, and staff perception.
Methods:
Methods: An evaluation of 24 ICU patients with our current balloon-based FMS was conducted with data collected twice per week. A total of 22 ICU patients were evaluated with the novel intrarectal SMK. Data was collected three times per week on only 18 patients due to early removal. Staff evaluations were completed (N=72).
Results:
Results: With the balloon intrarectal FMS, 1 patient experienced GI bleed with confirmed ulceration by colonoscopy. One GI bleed was observed with the novel intrarectal SMK, patient seen by the GI service, no scope completed. Bleeding stopped after SMK removal. Mean (±SD) days system in place: SMK 5.6 ± 3.5, FMS 10.5 ± 9.6. Peri-anal skin breakdown with redness but intact skin: SMK =3, FMS=2. Denuded or bleeding skin observed: SMK=0, FMS=3. Pressure injury (PrI) Stages 2 and 3 were 0 with SMK with 1 deep tissue injury (DTI) (chair for 7 hours). With the FMS, 2 Stage 2, 1 Stage 3 and 0 DTI were observed. No anal erosion with SMK; 2 with FMS. Staff reported leakage with SMK was none=19, small=33, moderate=9, large=6. Positive staff comments regarding the SMK included ease of insertion with the applicator. Negative comments were ease of dislodgement and gas valve clogging with no way to remove gas.
Discussion:
Discussion: The novel intrarectal SMK is an alternative to balloon based intrarectal FMS. Careful monitoring of both systems is needed to prevent GI and skin complications.
Trademarked Items:
References:

.png)