Case Series/Study
(CS-026) Utilizing a Novel Hydrogel for Maintaining Clean and Infection-Free Pin Sites in External Fixation During Limb Salvage: A Case Study.
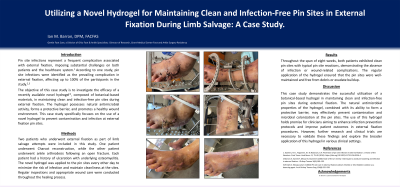
Pin site infections represent a frequent complication associated with external fixation, imposing substantial challenges on both patients and the healthcare system.1 According to one study, pin site infections were identified as the prevailing complication in external fixation, affecting up to 100% of the participants in the study.2,3
The objective of this case study is to investigate the efficacy of a recently available novel hydrogel*, composed of botanical-based materials, in maintaining clean and infection-free pin sites during external fixation. The hydrogel possesses natural antimicrobial activity, forms a protective barrier, and promotes a healthy wound environment. This case study specifically focuses on the use of a novel hydrogel to prevent contamination and infection at external fixation pin sites.
Methods: Two patients who underwent external fixation as part of limb salvage attempts were included in this study. One patient underwent Charcot reconstruction, while the other patient underwent ankle arthrodesis following an open fracture. Each patient had a history of ulceration with underlying osteomyelitis. The novel hydrogel was applied to the pin sites every other day to minimize the risk of infection and maintain cleanliness at the sites. Regular inspections and appropriate wound care were conducted throughout the healing process.
Results: Throughout the span of eight weeks, both patients exhibited clean pin sites with typical pin site reactions, demonstrating the absence of infection or wound-related complications. The regular application of the hydrogel ensured that the pin sites were well-maintained and free from debris or exudate buildup.
Discussion: This case study demonstrates the successful utilization of a botanical-based hydrogel in maintaining clean and infection-free pin sites during external fixation. The natural antimicrobial properties of the hydrogel, combined with its ability to form a protective barrier, may effectively prevent contamination and microbial colonization at the pin insertion sites. The use of this hydrogel holds promise for clinicians aiming to enhance infection prevention protocols and improve patient outcomes in external fixation procedures. However, further research and clinical trials are necessary to validate these findings and explore the broader application of this hydrogel in various clinical settings.
Trademarked Items: *FloraSeptic
References: 1. Kazmers, N.H., Fragomen, A.T. & Rozbruch, S.R. Prevention of pin site infection in external fixation: a review of the literature. Strat Traum Limb Recon 11, 75–85 (2016). https://doi.org/10.1007/s11751-016-0256-4
2. Moroni A, Vannini F, Mosca M, Giannini S (2002) State of the art review: techniques to avoid pin loosening and infection in external fixation. J Orthop Trauma 16(3):189–195
3. W-Dahl A, Toksvig-Larsen S (2004) Pin site care in external fixation sodium chloride or chlorhexidine solution as a cleansing agent. Arch Orthop Trauma Surg 124(8):555–558

.png)