Case Series/Study
(CS-050) Aseptically Processed Dehydrated Allograft Placental Membrane for Limb Salvage Reconstruction
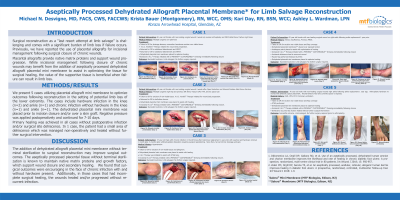
Surgical reconstruction as a “last resort attempt at limb salvage” is challenging and comes with a significant burden of limb loss if failure occurs. Previously, we have reported the use of placental allografts for incisional management following surgical closure of chronic wounds.
Placental allografts provide native matrix proteins and support wound progression. While incisional management following closure of chronic wounds may benefit from the addition of aseptically processed dehydrated allograft placental mini membrane to assist in optimizing the tissue for surgical healing, the value of the supportive tissue is beneficial when failure can result in limb loss.
Methods:
We present 5 cases utilizing placental allograft mini membrane to optimize outcomes following reconstruction in the setting of potential limb loss of the lower extremity. The cases include hardware infection in the knee (n=2) and ankle (n=1) and chronic infection without hardware in the knee (n=1) and ankle (n=1). The dehydrated placental mini membrane was placed prior to incision closure and/or over a skin graft. Negative pressure was applied postoperatively and continued for 7-10 days.
Results:
Primary healing was achieved in all cases without postoperative infection and/or additional surgical intervention. In 1 case, the patient had a small area of dehiscence which was managed non-operatively and healed without further surgical intervention.
Discussion:
The addition of dehydrated allograft placental mini membrane without terminal sterilization to surgical reconstruction may improve surgical outcomes in patients at risk for potential limb loss. The aseptically processed placental tissue without terminal sterilization is known to maintain native matrix proteins and growth factors, which support wound closure and secondary healing. We found that surgical outcomes were encouraging in the face of chronic infection with and without hardware present in the lower extremity at risk for amputation. Additionally, in those cases that had incomplete surgical healing, the wounds healed and/or progressed without recurrent infection.
Trademarked Items: Salera Mini membrane placental allograft: MTF Biologics ( Edison, NJ)
References: References
1. DiDomenico LA, Orgill DP, Galiano RD, et al. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves the likelihood and rate of healing in chronic diabetic foot ulcers: A prospective, randomized, multi-centre clinical trial in 80 patients. Int Wound J 2018; 15: 950-957.
2. Zelen CM, Orgill DP, Serena TE, et al. An aseptically processed, acellular, reticular, allogenic human dermis improves healing in diabetic foot ulcers: A prospective, randomized, controlled, multicenter follow-up trial. Int Wound J 2018: 1-9.

.png)