Case Series/Study
(CS-029) Application of Dehydrated Amniotic Membrane to Complex Diabetic Foot Ulceration: A Case Study
Methods:
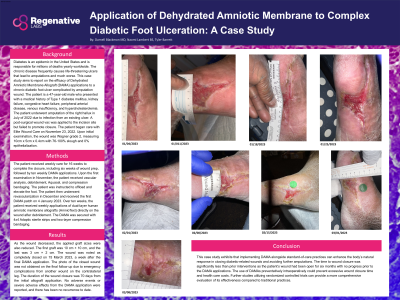
The patient received weekly care for 16 weeks to complete the closure, including six weeks of wound prep, followed by ten weekly DAMA applications. Upon the first examination in November, the patient received vascular analysis, debridement, Aquacel, and compression bandaging. The patient was instructed to offload and elevate the foot. The patient then underwent revascularization in December and received the first DAMA patch on 4 January 2023. Over ten weeks, the patient received weekly applications of dual-layer human amniotic membrane allografts (AmnioText) directly on the wound after debridement. The DAMA was secured with 4x4 Adaptic sterile strips and two-layer compression bandaging.
Results: As the wound decreased, the applied graft sizes were also reduced. The first graft was 10 cm × 10 cm, and the last was 3 cm × 2 cm. The wound was noted as completely closed on 15 March 2023, a week after the final DAMA application. The duration of the wound closure was 70 days from the initial allograft application. No adverse events or severe adverse effects from the DAMA application were reported, and there has been no recurrence to date.
Discussion: This case study exhibits that implementing DAMA alongside standard-of-care practices can enhance the body’s natural response in closing diabetic-related wounds and avoiding further amputations. The use of DAMAs preventatively intraoperatively could prevent excessive wound closure time and health care costs. Further studies utilizing randomized controlled trials are required for a more comprehensive evaluation of its effectiveness compared to traditional practices.
Trademarked Items:
References:

.png)