Laboratory Research
(LR-021) Know What’s in Your Membranes: Quantification of Factors in Placental Allografts
Although the favorable biological properties of the amnion and chorion have been explored for regenerative medicine indications, most published data on placental tissues does not relate the findings to allografts in their final, useable form. Furthermore, current reporting for eluted growth factor is not informative with respect to the factors available from the area of tissue, as applied, and may differ among techniques. During treatment with membrane product, the tissue is usually sterile, intact, and laid on a wound or treatment area. The factors available to the treatment area from the product so applied need to be presented such that the end user can extrapolate/determine the concentration in the tissue in their possession. We report here a method of quantifying these factors such that any membrane may be characterized, and data generated relates to the product in hand. Furthermore, use of this method facilitates evaluation of process improvements by providing a standardized methodology.
Methods:
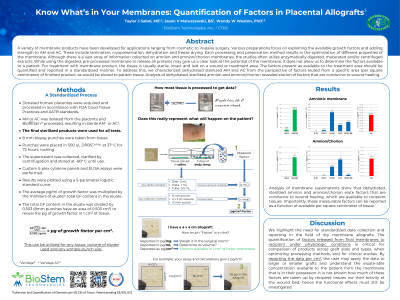
To address this, a standardized method of data collection, and formula for analysis, was developed and tested. We characterized dehydrated sterilized amniotic membrane and amnion/chorion factors eluted from a specific area of finished product, as would be eluted to patient tissue. The data from the elute was entered into the formula to yield a concentration per square centimeter of product.
Results:
Analysis of dehydrated, sterilized amnion and amnion/chorion revealed elution of factors that are conducive to wound healing. Factors eluted included VEGFR1, IL-1ra, HGF, PDGF-BB and HA, among others. Importantly, these measurable factors are reported as a function of available factor per square centimeter of tissue.
Discussion:
We highlight the need for standardized data collection and reporting in the field of membrane allografts. The characterization of factors released from final membranes per cm2 to recipient under physiologic conditions is critical for comparison of products across graft sizes and types, when optimizing processing methods, and for clinical studies. By reporting the data per cm2, the user may apply the data to larger or smaller grafts and understand the concentration available to the patient from the membrane that is in their possession. This method of characterization may be applied to any membrane size or composition. It is not known how much of these factors are taken up by recipient tissues nor their activity at the wound bed, but the data from functional studies will be optimized by this collection and reporting method.
Trademarked Items:
References: Sabol TJ, Tran GS, Matuszewski J, Weston WW. Standardized reporting of amnion and amnion/chorion allograft data for wound care. Health Sci Rep. 2022;5(5):e794. PMID: 36032519

.png)